Abstract
Objectives Since the introduction of tyrosine kinase inhibitors (TKIs), chronic myeloid leukemia (CML) has evolved from a devastating disease to manageable due to the availability of oral medications. Recent reports indicate an improvement in life expectancy attributed to TKIs. Our objective is to investigate participation in clinical trials from a global perspective to identify regions lacking representation in these pivotal trials. In addition, we inquire into TKI drug approvals and a country's socio-demographic index (SDI).
Methods A search was conducted on ClinicalTrials.gov (CT.gov) using the key words "Chronic Myeloid Leukemia". Additional filters were applied to only include phase 2 and phase 3 trials. This query yielded 702 trials, of which 212 had results posted. Fifty-two of these trials used TKIs to treat CML. Trials reporting single-country participation (9; primarily in China, Japan, US, and Turkey) and early termination (13) were excluded. Information on the participating sites for the 30 eligible trials were extracted from CT.gov. CML incidence and disability-adjusted life years (DALYs) from 1999 to 2019 were collected per World Bank regions and individual countries from the Institute of Health Metrics and Evaluation (IHME) database then categorized by SDI. The difference in DALYs over the 10-year period and locations that participated in clinical trials were mapped. Z-statistics were used to assess the difference in proportions of regional DALYs in relation to the proportion of locations that participated in the trials. Global drug approvals for imatinib, nilotinib, bosutinib, ponatinib, and asciminib were collected from their respective pharmaceutical company. Dasatinib data is pending.
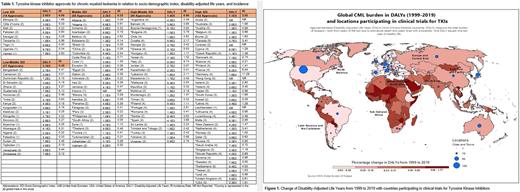
Results The 30 trials represented 4 imatinib, 11 dasatinib, 8 nilotinib, 3 bosutinib, 3 ponatinib, and 1 asciminib trials. There were 755 locations with investigational sites that participated in these trials. Sub-Saharan African (SSA) had only 11 participating locations (1.5%, all in South Africa). Latin America and Caribbean (LAC) had 46 (6.1%) locations distributed across 10 countries. South Asian (SA) countries were represented by 13 (1.7%) locations, all within India. Europe and Central Asia (ECA) had the 344 locations (45.6%) across 30 countries. The Middle East and North Africa (MENA) had 19 (2.5%) locations in eight countries, while East Asia and Pacific (EAP) had 121 (16.0%) locations with participating sites. In North America (NA, including US and Canada), 201 (26.6%) locations had sites that participated in the trials. Africa, the Middle East, and Latin America had significantly lower representation in the clinical trials than Europe, Asia, or the US and Canada. CML incidence rates reported by IHME from highest to lowest per 100,000 cases were ECA: 3.01, NA: 1.7, SA: 0.62, MENA: 0.57, EAP: 0.45, SSA: 0.43, and LAC: 0.35. DALYs, from highest to lowest were SA: 4.16x105, SSA: 2.10x105, EAP: 1.25x105, ECA: 1.22x105, MENA: 7.58x104, LAC: 6.27x104, and NA: 3.66x104. Calculated Z-scores were SA -56.13 (p<.001), SSA -43.74 (p<.001), EAP 10.24 (<.001), ECA 1.91 (p=.003), MENA -30.93 (p<.001), LAC 2.93 (p= .003), and NA 2.01 (p=.044) Despite ECA and NA having the highest incidence of CML, countries in SA and SSA had significantly more DALYs and the least regional representation in trials (Figure 1). NA and ECA had more locations with investigational sites. Table 1 shows DALYs and incidence overtime for these regions. Of the TKIs collected for drug approvals, there are 142 approvals in 40 High SDI countries, 90 approvals in 30 High-Middle SDI countries, 68 in 31 Middle SDI countries, 21 in Low-Middle SDI countries, and 14 approvals in 9 Low SDI countries. None of the Low-Middle and Low countries yielded >2 approvals.
Conclusion The availability of global representative trials is important, especially in regions with underserved populations and greater disease burden. SA, SSA, and MENA had significantly lower representation in 30 trials that investigated TKIs in CML, while experiencing greater burden in DALYs compared to other regions. In addition, findings show that lower SDI countries had fewer approvals for TKIs leading to further disparities. This reveals a disparity that is important to address.
Disclosures
Cortes:Forma Therapuetic: Consultancy; Abbvie: Consultancy, Research Funding; Gilead: Consultancy; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Kartos: Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Bristol Myers Squibb: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.